
Funded with an ERC Starting Grant from the European Research Council
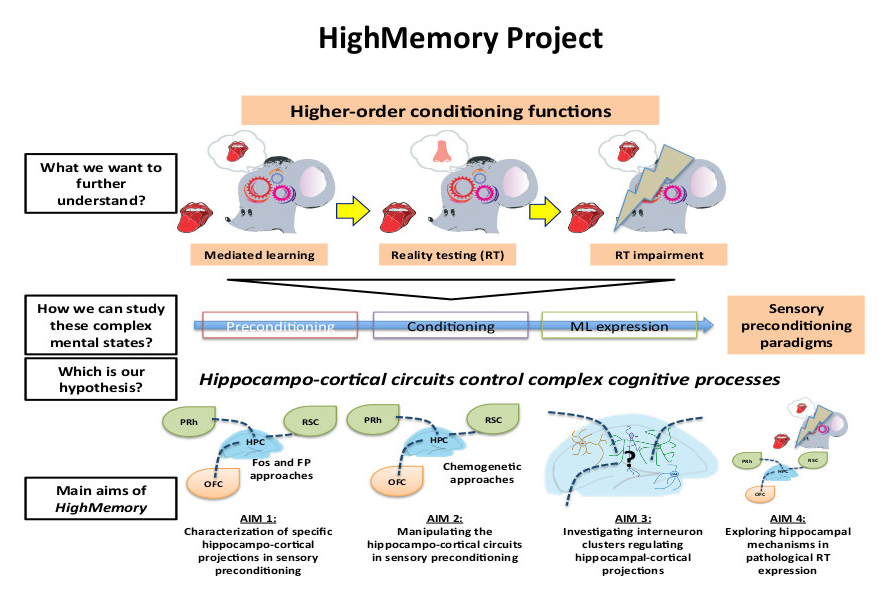
Animals and humans adapt to changes in the environment encoding and storing previous experiences. Although associative learning involving a reinforcer has been the major focus in the field of cognition, other forms of learning are gaining popularity as they are likely to be both more significant and frequent in human daily choices. Indeed, associations between non-reinforcing stimuli represent the most evolutionarily advanced way to increase the chances of predicting future events and adapting an individuals' behaviour. Animals are also able to utilise these higher-order conditioning processes, but more research is needed to understand how the brain encode and store these complex cognitive processes.
The HighMemory project looks at the role of hippocampo-cortical circuits in higher-order conditioning processes in mouse models. These processes explain why individuals are very often repulsed or attracted by stimuli (people, places, sounds), which have no intrinsic repellent or appealing value and have never been explicitly paired with negative or positive outcomes. A possible explanation for these "ungrounded" aversions or repulsions is that these stimuli have been incidentally associated with other directly reinforced cues. This is called higher-order conditioning or mediated learning (ML). However, with an increased number of incidental associations, the subjects acquire more information, allowing separation between the true saliences of two different stimuli. Therefore, with increased training, ML evolves into what researchers define as "reality testing" (RT). Importantly, these behavioural processes involve the hippocampus, are characterised by defined and accessible phases and involve several brain regions, making them perfect models for studying tight behaviour regulation by hippocampo-cortical projections. By using cutting-edge genetic (viral and chemogenetic techniques), Ca2+ imaging, and mouse behavioural (sensory preconditioning) approaches, HighMemory is at dissecting and characterizing, at the macro- (brain regions), meso- (cell sub-types), and micro-scales (activity changes), the causal involvement of hippocampo-cortical projections in higher-order cognitive processes, from the formation of ML, the transition to RT, and the expression of these distinct mental states. Notably, we hope that HighMemory will provide important information to help us better understand and tackle the physiology of complex cognitive processes and mental disorders such as psychotic-like states.
Funded by the Retos Call 2018 from the Spanish State Research Agency (AEI).
Alzheimer's disease (AD) is a neurodegenerative process characterised by molecular alterations, such as the presence of senile plaques, and behavioural deficits including cognitive impairments. AD has also been associated with numerous alterations of brain bioenergetic processes, including changes in astroglial metabolism and mitochondrial functions. However, the precise mechanisms of these dysfunctions and their impact on the pathophysiology of AD remain unknown.
Type-1 cannabinoid (CB1) receptors, are one of the main components of the endocannabinoid system (ECS), and control various intercellular processes such as cell metabolism and synaptic plasticity. Importantly, they have been linked to the pathophysiology of AD. Indeed, alterations of different ECS components have been described in both, animal models of AD and human patients. Moreover, in vitro and in vivo pharmacological activation of CB1 receptors effectively reduces the neurotoxic effects of amyloid-B peptide and counteracts the cognitive impairment found in AD mouse models. Although accumulating evidence points towards CB1 receptors playing a role in AD pathophysiology, further research is needed to decipher the specific mechanisms linking ECS and AD.
CB1 receptors are functionally present in different cell-types (neurons and astrocytes) in both the plasmatic and mitochondrial membranes. Indeed, cannabinoids acting on mitochondrial CB1 receptors (mtCB1) affect brain mitochondrial respiration and control behavioural processes. However, whether cell-type- or mtCB1-dependent mechanisms can participate in a pathological condition such as AD has not yet been explored. Therefore, in this project, it is hypothesised that CB1R-dependent cell-type specific bioenergetic processes participate in AD.
The BECALM project is very important as it should provide us with a better understanding of how the ECS in general, and CB1 receptors in particular, might be considered a potential therapeutic targets for AD for several reasons; Firstly using animal models, it involves in depth research into the impact and importance of bioenergetic processes on the development of molecular and behavioral AD phenotypes. By focusing on individual and gender variability it will help to identify specific bioenergetic alterations underlying possible differences in vulnerability towards cannabinoid-induced improvements in molecular or behavioural deficits; secondly, this proposal should provide potential translational observations thanks to the use of mouse models; thirdly, by targeting specific brain regions and cell-types, this project aims to provide a thorough functional mapping of CB1 receptors and bioenergetic involvement in AD at the macro- (brain region), meso- (cell type) and micro-scale (subcellular localisation). In conclusion, by exploring novel and promising scientific avenues, this project will substantially contribute towards the comprehension of the mechanistic basis of AD.
Coordinator:
Arnau Busquets(ELIMINAR)
Tel:
93 3160390
Dr. Aiguader, 88, 2ª Planta
08003 Barcelona
© Institut Hospital del Mar
d'Investigacions MèdiquesLegal Notice and Privacy Policy | Cookie Policy | Site Index | Accessibility | Find Us | Contact